In an internal combustion engine, air at atmospheric pressure and a temperature of about is compressed in the cylinder by a piston to of its original volume (compression ratio = 9.0). Estimate the temperature of the compressed air, assuming the pressure reaches 40 atm.
In order to watch this solution you need to have a subscription.
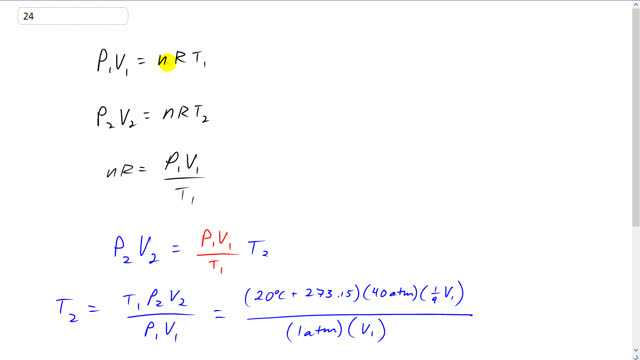
This is Giancoli Answers with Mr. Dychko. Initially in the cylinder you have some initial pressure atmospheric pressure, p1, times some initial volume, v1, which we don't know. And it equals the number of moles of the gas in there times the universal gas constant times the initial temperature expressed in kelvin. And then after the compression happens. You have some new pressure p2 times some new volume v2 equals n R times t2. And this n does not have a subscript because it's the same molar quantity of gas in both cases. So, n R is p1 v1 over t1 if you divide both sides of this by t1. And then we can substitute this in place of n R in the second formula. So, we have p2 v2 equals p1 v1 over t1 times t2. And then we can solve for t2 by multiplying both sides of this by t1 over p1 v1. And the p2 v2 gets multiplied by t1 over p1 v1. And the t2 gets isolated and we switch the sides around. And so the temperature after compression is going to be the initial temperature, 20 degree Celsius plus 273.15 to convert it into kelvin times 40 atmospheres after compression, p2, times the new volume which is 1/9 times the initial volume and divide that by the initial pressure of 1 atmosphere times the initial volume, v1. The v1's cancel. And this works out to 1302.89 kelvin. And if you take away 273.15 we can write this in Celsius. And this kind of explains why diesel engines don't need spark plugs because after compression the temperature is so high that this can ignite a diesel fuel just as a result of the temperature increased because of compression.
