You have a sample of water and are able to control temperature and pressure arbitrarily.
- Using Fig. 13–22, describe the phase changes you would see if you started at a temperature of , a pressure of 180 atm, and decreased the pressure down to 0.004 atm while keeping the temperature fixed.
- Repeat part (a) with the temperature at . Assume that you held the system at the starting conditions long enough for the system to stabilize before making further changes.
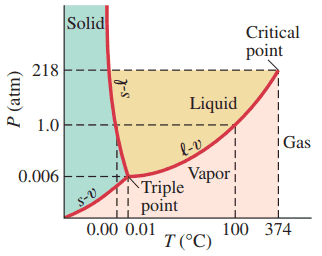
- liquid to vapour
- liquid to solid to vapour
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. At a temperature of 85 degrees Celsius where somewhere along this vertical line and a pressure of, what is it? 180 atmospheres, is way up here somewhere. So, here's 180 atmospheres on the vertical axis and 85 degrees Celsius on the horizontal axis. That put us starting at this point here. It is a liquid anywhere anywhere to the left of this critical temperature, and to the right of this line here is going to be liquid. So, it's liquid all in here. So, liquid, liquid, liquid as the pressure decreases until it gets to this point where it changes phase into vapor. And this triple point is at 0.006 atmospheres and we're going to go down to a pressure of 0.004 atmospheres. So, we are going to get below this dotted line here, to just about here say, and so that definitely puts us in the vapor region. So, we'll see a transition from liquid into vapor. And part b, it says, we'll have a temperature of 0 degrees. OK. And we'll start away up there the same high pressure. Let's do it in blue this time. So, 0 degrees, high pressure, that puts us in the, just barely in the liquid region because this line here is separating solid from liquid kind of curves away to the left as you get to lower and lower, or, yeah, as you get higher and higher it kind of curves to the left here. So, we're gonna start in liquid here at 180 atmospheres and 0 degrees Celsius. And as we go down further, once we get to regular sea level standard temperature and pressure conditions of 1 atmosphere and 0 degrees Celsius, we're going to have solid. And then it's solid, solid, solid. And then, well, my drawing isn't perfect here. But finally we get to this point here where it's 0.004 atmospheres, and it crosses below this line and transitions from solid to vapor. So, liquid to solid to vapor is what you'd see.
