How many moles of water are there in 1.000 L at STP? How many molecules?
In order to watch this solution you need to have a subscription.
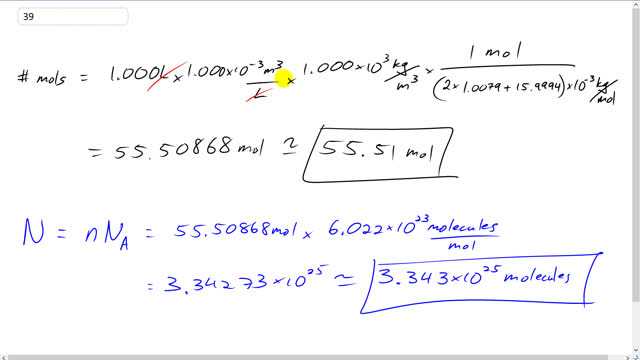
This is Giancoli Answers with Mr. Dychko. The number of moles of water is going to be 1 liter times 1 times 10 to the minus 3 cubic meters per liter to convert this into cubic meters. And then multiplied by the density of water, and we assume it's fresh water, so, that's 1.000 times 10 to the 3 kilograms per cubic meter and then multiplied by 1 mole for every this many kilograms. So, we're basically, you know, we're multiplying by the reciprocal the molar mass. So, we have 1 mole for every 2 times 1.0079, that's the number of grams per grams per mole of hydrogen, and there's 2 hydrogens in a water molecule, it's H2O. And it's gram, so, that means that's why we have this times 10 to the minus 3 outside here to convert it to kilograms. And then plus the a single oxygen atom which is 15.9994. And this all works out to 55.51 moles. And the number of molecules will be the number of moles multiplied by Avogadro's number which is the number of molecules per mole. And so we take 55.50868 moles times 6.022 times 10 to the 23 molecules per mole. And we get 3.343 times 10 to the 25 molecules.
Why cant you use ideal gas law
Hello rjosaphiv, thank you for the question. It makes sense that "STP" makes you think of gases, but as it turns out here - water is not a gas at Standard Temperature and Pressure. The standard temperature is , or , at which point water is liquid, so our calculation uses the density of water as a liquid.
All the best,
Shaun
