If the air pressure at a particular place in the mountains is 0.80 atm, estimate the temperature at which water boils.
In order to watch this solution you need to have a subscription.
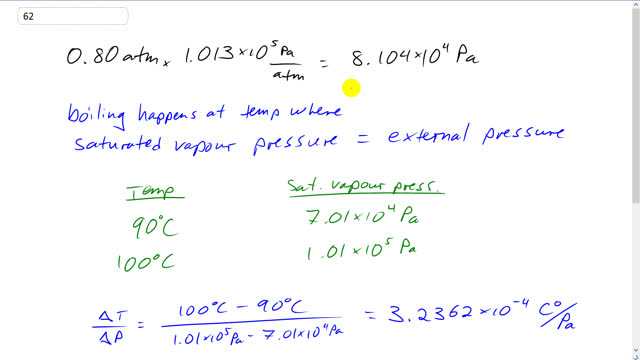
This is Giancoli Answers with Mr. Dychko. Let's convert this pressure of 0.80 atmospheres into pascal so that we can look at table 13-3 and compare it because all of our pressures on this table are in pascals or torr but we're not gonna use tor because that's just silly, that's millimeters of mercury. And so we have 0.8 atmospheres times 1.013 times 10 to the 5 pascals per atmosphere which gives 8.104 times 10 to the 4 pascals. So, boiling happens when the saturated vapor pressure equals the external pressure. So, whenever we have this external pressure for the saturated... So, whatever, you know. So, we have this table here and it's giving us some temperatures and it's giving us the saturated vapor pressures that happen at those temperatures. And when the saturated vapor pressure is this pressure, then we have boiling. Now, this number doesn't appear anywhere in the table, it's somewhere between these two pressures. So, somewhere between 90 and 100 degrees Celsius. And to figure out exactly where, we're going to assume that the pressure varies linearly with temperature in this interval between 90 and 100 degrees Celsius which is sort of a wordy way of saying that we're going to figure out the rate of change of temperature with respect to pressure by taking the difference in temperature here, 100 minus 90, and divide by the difference in pressure. And we get the number of degrees, Celsius degrees, that the temperature goes up per pascal. And so taking the difference in temperature divided by the difference in pressure gives us 3.2362 times 10 to the minus 4 Celsius degrees per pascal. This is the slope of the line if we were to graph temperature versus pressure. So, pressure would be on this axis and temperature would be on this axis here. And here's one point that we know at... Other way around. Celsius and pascals. So, this would be 90 degrees here and this would be 100 degrees here and would be 7.01 times 10 to the 4 pascals and this would be 1.01. And so the point we're trying to find is somewhere in here. And we know what the pressure is, it's this pressure. And our job is to figure out at what temperature that happens. So... Strictly speaking, I guess the way I've done this is as I put the axes the way, I first wrote them down which is like this where you have this rise of a run. So, the rise is a change in temperature and the run is change in pressure. So, this would be 90 and this would be 100 and this would be 7.01 and this would be 1.1. OK. Anyway. So, we found the slope now. So, to find any other temperature, we'll take some temperature that we know such as 90 degrees. And then add to that the rate of change of temperature with pressure times the change in pressure. So, we take this 90 degrees and that to that the 3.2362 times 10 to the minus 4 Celsius degrees per pascal and times by the change in pressure between what we want and the pressure for which we have some data. And then we end up with 94 degrees Celsius must be this temperature here.
