
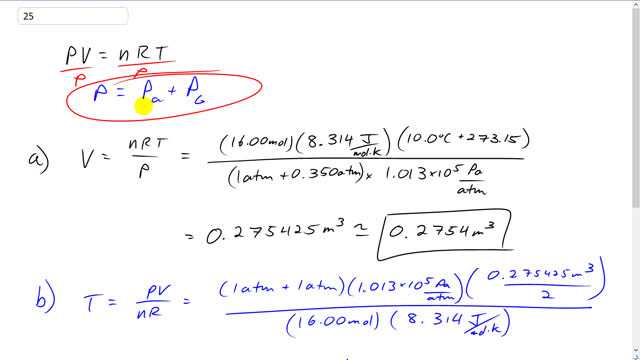
If 16.00 mol of helium gas is at and a gauge pressure of 0.350 atm, calculate
- the volume of the helium gas under these conditions, and
- the temperature if the gas is compressed to precisely half the volume at a gauge pressure of 1.00 atm.
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. We're gonna solve the ideal gas law for volume by dividing both sides by p for pressure. And we need to keep in mind that pressure is absolute pressure, so it's atmospheric pressure plus the gauge pressure and this question tells us the gauge pressure of 0.35 atmospheres. And so we have to add 1 atmosphere to that to get the absolute pressure. So, v is n R T over p. So, that's the number of moles, 16, times the ideal gas constant, 8.314 joules per mole kelvin times the temperature written in kelvin. So, it's 10 degrees Celsius plus 273.15. Divided by the absolute pressure of 1 plus 0.35 atmospheres. And then you have to convert those atmospheres into pascals by multiplying by 1.013 times 10 to the 5 pascals per atmosphere. And then we get 0.2754 cubic meters must be the volume. And then if the container is compressed so that if the volume is now half what it was before. So, this is the answer from part a, 0.275425 cubic meters divided by 2. And the pressure is increased so that the gauge pressure is 1 atmosphere. And in which case the absolute pressure is the atmospheric pressure plus that. So, that's 2 atmospheres in total for absolute pressure. And then convert that into pascals and then divide by the number of moles which is the same, we haven't changed the quantity of helium inside the container, it's still 16 moles times by the universal gas constant. And then you get, well, 209.7407 kelvin. And if you take away 273.15, that gives you your answer in Celsius. So, we have negative 63 degrees Celsius.