Two isotopes of uranium, and (the superscripts refer to their atomic masses), can be separated by a gas diffusion process by combining them with fluorine to make the gaseous compound . Calculate the ratio of the rms speeds of these molecules for the two isotopes, at constant . Use Appendix B for masses.
In order to watch this solution you need to have a subscription.
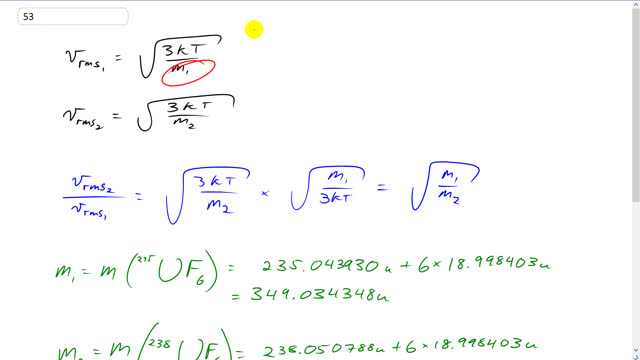
This is Giancoli Answers with Mr. Dychko. The rms speed of the first isotope is going to be square root of 3 times Boltzmann's constant times the temperature divided by the molecular mass of that first isotope. And V rms2 is going to be square root of 3 K T over the second molecular mass. So, the ratio of speeds is going to be square root of 3 K T over m2 multiplied by the reciprocal of V rms1. So, you know, dividing by V rms1 is the same as multiplying by its reciprocal. So, it'll multiply by the reciprocal because it's easier to see how things cancel. And this works out to square root m1 over m2. So, the molecular mass of the first isotope is going to be with uranium 235 times with along with 6 fluorine atoms. And looking at appendix B, you can see the atomic mass of uranium 235 is 235.043930 atomic mass units plus 6 times 18.998403 atomic mass units which gives this answer for the molecular mass for this isotope here. Now, when you have uranium 238, that's the same calculation except you have 238.050788 atomic mass units. That gives this. And then you could convert those into kilograms if you wanted to but you'd be multiplying both things by the same conversion factor and that would just cancel anyway when you divide. So, there's no need to do that. So, we have V rms2 over V rms1 is square root 352.041206 atomic mass units divided by 349.034348 atomic mass units which is 1.0043 is the ratio of the rms speeds.
in the blue equation it shows the square of m1/m2 but when you solve it you put the square of m2/m1...can you explain why you did not flip Vrms2/Vrms1 but did so for the left side of the equation?
Hi merkinthedark, thank you for spotting this error. To get the final answer I should have solved for which would give , and then plugged the numbers as shown in the video. The final answer would be correct for . If we pause to apply the "reality check" to the answer (by asking yourself "self, does this number make sense?") we would expect the isotope with less mass to be faster than the heavier isotope since the speed depends only on temperature (which is the same for each in this question), and inversely proportional to mass (meaning smaller mass gives higher speed) according to . This means, with the numbers plugged in the way they are in the video, this should be solving for , not . I've flagged this video for a retake one day and made a note in the quick answer section.
All the best,
Mr. Dychko
