A scuba tank is filled with air to a gauge pressure of 204 atm when the air temperature is . A diver then jumps into the ocean and, after a short time on the ocean surface, checks the tank’s gauge pressure and finds that it is only 191 atm. Assuming the diver has inhaled a negligible amount of air from the tank, what is the temperature of the ocean water?
In order to watch this solution you need to have a subscription.
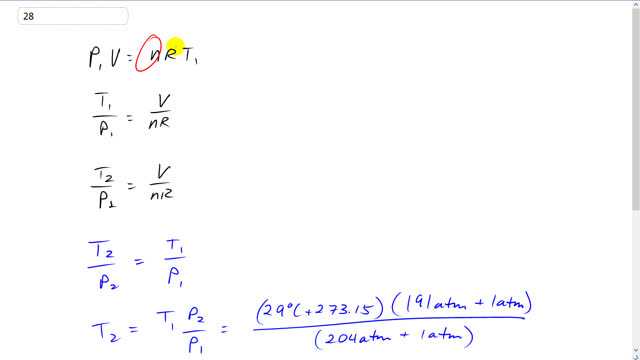
This is Giancoli Answers with Mr. Dychko. When the scuba tank is on the divers back when he's on the boat, it has some pressure p1 times the volume of the tank times the number of moles of air inside at times the universal gas constant times the temperature on the boat in kelvin. And we're going to divide both sides by n R and v. And we, I'm sorry, n r and p1 I should say. And we're going to have the n R's cancel on this side and left with t1 over p1. On the other side we'll have v over n R because the p1's will cancel there. And we get t1 over p1, after we switch the sides around, is the volume of the tank divided by the number of moles of air times the universal gas constant. And then once the person jumps into the water, there's gonna be a new temperature and a new pressure but the volume of the tank will stay the same because it's a rigid metal tank and the number of moles of gas will be the same too because we're told to neglect, you know, the few breaths of air that the person might have taken on route from the boat to the water. So, both of these fractions equal the same thing. So, that makes them equal to each other. So, t2 over p2 equals t1 over p1. And we can solve for t2 the temperature in the ocean by multiplying both sides here by p2. And temperature in the ocean is the temperature on the boat times the pressure gauge pressure is, sorry, the gauge pressure plus the atmospheric pressure to get the absolute pressure in the ocean divided by absolute pressure on the boat. So, we have 191 atmospheres gauge pressure in the ocean plus 1 atmosphere to get the absolute pressure. And then divide that by the gauge pressure on the boat, 204 atmospheres plus 1 atmosphere to get the total absolute pressure. And times by the temperature on the boat in kelvin. So, it's 29 degrees Celsius plus 273.15. That gives 2082.989 kelvin. And this number is precise to the ones place which makes this total have 3 significant figures. And that means this number here has 3 significant figures and it's therefore precise to the ones place. And then when we take this difference here and subtract 273.15 to turn it into Celsius, the answer here has to be precise only to the ones place because that's the most precise digit in this number. And so that means our answer is precise to the ones place and were forced to round it to 10. 10 degrees Celsius is our answer, that's the temperature of the ocean.
