A sealed test tube traps of air at a pressure of 1.00 atm and temperature of . The test tube’s stopper has a diameter of 1.50 cm and will “pop off” the test tube if a net upward force of 10.0 N is applied to it. To what temperature would you have to heat the trapped air in order to “pop off” the stopper? Assume the air surrounding the test tube is always at a pressure of 1.00 atm.
In order to watch this solution you need to have a subscription.
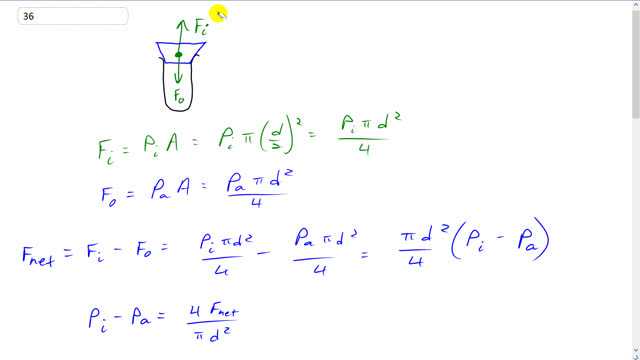
This is Giancoli Answers with Mr. Dychko. There's a force upwards on this test tube stopper due to the air pushing on it from the inside. So, we'll call that F subscript i for inside, the force upwards due to the air inside. Then there's a force downwards due to the air outside, we'll call that Fo for outside. And the inside force is going to be the pressure times the area. And the area is π times the radius of a stopper squared or the diameter over 2 squared. And so that means pressure inside times π times diameter squared over 4. And the force pushing down from the outside is atmospheric pressure times area π d squared over 4 so, now, I guess I've drawn this as a tapered stopper, and so you might think that the area outside is bit bigger but it doesn't quite work like that because the amount that this is exceeding the inside area. Well, there's also atmospheric pressure pushing up on this beveled side here. And the projected area of this beveled part is the same as this area up here. Anyway. So, it cancels out. So, never mind the fact that it's tapered, doesn't matter. So, the areas are effectively the same. So, the net force on the stopper is the force due to the air inside minus the force due to the air outside upwards minus the down force. And substituting for each of those, pressure inside times π d squared over 4 minus atmospheric pressure outside times π d squared over 4 which we can factor out that π d squared over 4 and just multiply it by the difference in pressures inside minus outside. And then you can solve for just Pi minus Pa by multiplying this by 4 over π d squared. And then multiply this by 4 over over π d squared as well. And you get this. And then solve for the pressure inside by adding atmospheric pressure to both sides. So, the pressure inside has to be 4 times the net force divided by π d squared plus the pressure outside, atmospheric pressure. And we know that the atmospheric pressure times volume equals n R T1. This is the initial case inside the test tube. So, inside the test tube at T1, it begins with atmospheric pressure. And then we can divide both sides by atmospheric pressure and n and R. And on the right hand side we have T1 over Pa. And on the left hand side we have V over n R. So, T1 over Pa equals V over n R. And then after you increase the temperature inside the test tube you'll have a new temperature, and then a new pressure, we'll call it P inside. This is also the pressure inside but it happens to also be Pa. So, let's just write it as Pa. And then that's, this is also going to equal V over n R because the test tube volume hasn't changed, it's a rigid tube, and the number of moles of air inside hasn't change because the tube is sealed. And so since each of these things equals V over n R, they're equal to each other. So, we have T2 over pressure inside when it pops equals T1 over atmospheric pressure. And solve for T2 by multiplying both sides by Pi. And so we have, the temperature when it pops is T1 times the pressure inside when it pops divided by atmospheric pressure. And we substitute from way up here for the pressure inside down here. So, that's 4 times F net over π d squared plus atmospheric pressure. And divide each of these terms by the denominator and then this term becomes 1. And this term has this Pa in the bottom. So, we have T1 times for F net over π d squared Pa plus 1. And that's 18 degrees Celsius plus 273.15 initial temperature times 4 times 10 newtons of net force needed to pop the stopper off divided by π times 1.5 times 10 to the minus 2, convert those centimeters into meters square that diameter, times with 1.013 times 10 to the 5 pascals atmospheric pressure and then plus 1. And you get 453.793 kelvin. And after you subtract 273.15, you get the answer in Celsius. And this number is precise to the ones place, so it has three significant figures after you add them. And so this has three significant figures making it precise to the ones place, and so this difference is precise to the ones place. And that means we round it to 181 degrees Celsius. And then the stopper will pop off.
