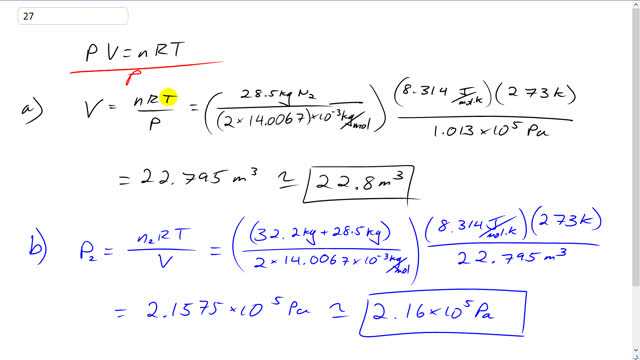
A storage tank at STP contains 28.5 kg of nitrogen .
- What is the volume of the tank?
- What is the pressure if an additional 32.2 kg of nitrogen is added without changing the temperature?
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. We can solve the ideal gas law for volume by dividing both sides by p. And we have the volume is the number of moles of the gas times the universal gas constant times the temperature in kelvin divided by the absolute pressure. So, the number of moles of nitrogen is 28.5 kilograms divided by the molar mass expressed in kilograms per mole. And so we look in the back cover of the textbook and you see 14.0067 written in this little square that has nitrogen, and it has atomic mass 7 right between oxygen and carbon. And there's 2 nitrogen atoms per molecule. So, we have this times by 2 here. And that's a grams per mole. And then multiply by 10 to the minus 3 kilograms per mole so that the kilograms here cancel with the kilograms there. So, that takes care of the n, the number of moles. Then we have to multiply by 8.314 joules per mole kelvin and then times by the temperature. Now, since this is STP standard temperature and pressure, the temperature is 273 kelvin. Then we divide by 1 atmosphere 1.013 times 10 to the 5 pascals. And that gives 22.8 cubic meter must be the volume of the container. Now, in part b we're going to add an additional 32.2 kilograms. So, since it's additional, that means the total mass is 32.2 plus the initial 28.5 kilograms of nitrogen. And the volume is gonna stay the same for the container, we're just changing the number of moles. And the temperature is the same. So, we're dividing this by 2 times 14.0067 times 10 to the minus 3 kilograms per mole and then times by universal gas constant and times by the standard temperature and divide by the volume of the container that we found out in part a, that's 22.795 cubic meters. And we get new pressure of 2.16 times 10 to the 5 pascals. And we expected this to be higher than it was before so that makes sense because there's now more molecules in here, so, we'd expect more collisions between them and more pressure.
