Estimate the number of
- moles and
- molecules of water in all the Earth’s oceans. Assume water covers 75% of the Earth to an average depth of 3 km.
In order to watch this solution you need to have a subscription.
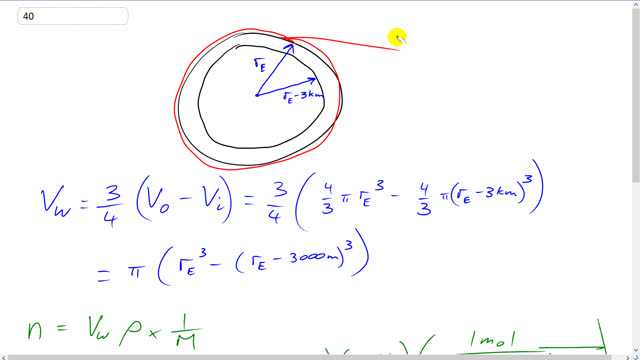
This is Giancoli Answers with Mr. Dychko. We need to find the volume of this, a bit of a shell here this is gonna be the volume of the earth which is this outer shell. And then subtract the volume that's inside here this is the radius of the earth minus the 3 kilometer depth of the oceans here. And so the volume of water is going to be 3/4 of this shell here. And so that's 3/4 times the outside volume minus the inside volume. So, that's 3/4 times 4/3 π times radius of the earth cubed minus 4/3 π times radius of the earth minus the kilometers cubed. And 4/3 can get factored out and it cancels with the 3/4. So, we just have π factored out times radius of the earth cubed minus radius the earth minus 3,000 meters cubed. So, the number of moles will be the volume of the water times the density of water, and that's sea water. Most water is sea water, so we'll just assume it's all sea water. And then times by the reciprocal of the molar mass. So, that's number of mole or 1 mole for every how many kilograms. So, that's π times 6.38 times 10 to the 6 meters radius of the earth, cubed, minus 6.38 times 10 to the 6 meters minus 3,000, cubed, times 1,025 kilograms per cubic meter density of seawater. And so now the cubic meters cancel there. And then we have 1 mole for every 2 times the molar mass of hydrogen, 1.00794 grams plus 15.994 molar mass of oxygen. And since these are grams per mole, we go times 10 to the minus 3 to convert it into kilograms. And this makes about 7 times 10 to the 22 moles. No sense having more than one significant figure in here because there's a lot of approximations involved, first of all that all the water is seawater and plus these radi which just averages I mean there's actually bulges near the equator, it's not an actual sphere. And then define the number of molecules that would represent, take the number of moles multiplied by Avogadro's number, 6.022 times 10 to the 23 molecules per mole. And we get about 4 times 10 to the 46 molecules.
