What is the dew point if the humidity is 65% on a day when the temperature is ?
In order to watch this solution you need to have a subscription.
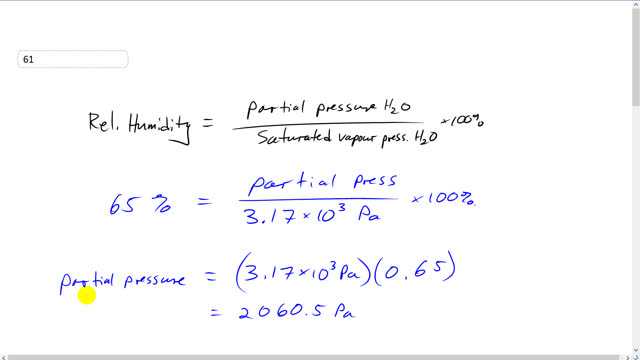
This is Giancoli Answers with Mr. Dychko. The first thing we need to do is figure out what the partial pressure of water is and at this temperature with 65% humidity. And then once we know what that partial pressure is, we figure out what temperature would that pressure be, saturated vapor pressure, and that will be the temperature which the, you know, the water is gonna come out of the air and form, condense into dew. So, that temperature would be the dew point. So, relative humidity is partial pressure divided by saturated vapor pressure and at 25 degrees Celsius saturated vapor pressure of water is 3.17 times 10 to the 3 pascals. And since relative humidity is 65% that means the partial pressure of water is going to be 3.17 times 10 to 3 pascals multiplied by 0.65. So, rearranging this a little bit, multiplying by this and dividing by that, and you get this line here. So, it's 2060.5 pascals is the partial pressure. And when we look at this table 13-3 on page 379, you won't find a number that's particularly close to 2 times 10 to the 3 pascals. And so we need to do what's called a linear interpolation which is a fancy word for saying let's assume that the, you know, if you were to graph saturated vapor pressure versus temperature between these two known points that do appear in the table, we'll assume that the, going from one to the other follows this linear path. So, if we can figure out the slope of this line and also figure out b, where it crosses the y-intercept, we can make a function y equals mx plus b, that will tell us what the saturated vapor pressure is in terms of the temperature. So, let's do this. Let's figure out the slope. So, that's gonna be the Y coordinate at this point minus the Y coordinate at this point, that's the rise. And divide that by the run. So, that's the horizontal difference between these two points. So, that's x2 minus x1. And so we know what the saturated vapor pressure is at 20 degrees Celsius. So, you put that in, 2.33 times 10 to the 3 pascals. So, the coordinates of this point are 20 degrees Celsius, 2.33 times 10 to the 3 pascals. And then take that Y coordinate minus the Y coordinate at this point that we know in the table, that's 1.71 times 10 to 3 pascals, and that happens at 15 degrees Celsius. And this gives a slope of 124 pascals of saturated vapor pressure for every Celsius degree. And then figure out b, the y-intercept. And, you know, subtract mx from both sides there. And hit b is y minus mx. And let's take one of the points that we know, 2.33 times 10 to the 3 pascals minus the slope that we just found times the temperature at which this pressure happens, 20 degrees Celsius. And we get negative 150 pascals. So, our equation for saturated vapor pressure versus temperature between 15 and 20 degrees Celsius is y equals 124 pascals per Celsius degree times x minus 150. So, we can solve this for x which is the temperature by adding this to both sides and then dividing by the slope. So, we have x equals y plus 150 pascals divided by 124 pascals by Celsius degree. And now we plug in this, what is the partial pressure at 25 degrees Celsius, and figure out at what temperature this becomes saturated vapor pressure and that's what this equation is, this Y coordinate is the saturated vapor pressure. So, we say that's what the... So, we say the saturated vapor pressure is 2,060.5 pascals and then plus 150 divided 124. And we get about 18 degrees Celsius must be the temperature at which the partial pressure becomes a saturated vapor pressure, and that's the dew point.
