A helium-filled balloon escapes a child’s hand at sea level and . When it reaches an altitude of 3600 m, where the temperature is and the pressure only 0.68 atm, how will its volume compare to that at sea level?
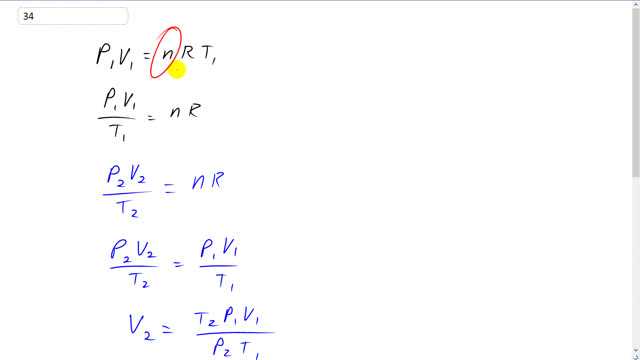
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. When the helium balloon is in the child's hand, the pressure is an atmospheric pressure, P1, and it has some initial volume, V1, and that equals n, the number of moles of air inside the balloon times the universal gas constant times the temperature at sea level. And we can divide both sides by T1 and get P1 V1 over T1 is n R. And then when the balloon is way up in the atmosphere, there's still gonna be the same number of moles of gas in it, but there's gonna be a different pressure, different volume and a different temperature. And so we have P2 V2 over T2 is the same n R but since both of these equal and n R, they're equal to each other and so that's what we write here. And then we can solve for V2, multiply both sides by T2 over P2. So, V2 is T2 P1 V1 over P2 T1. And comparing it to the initial volume, good way to compare it is to divide it maybe and see what the fractional changes. And so that V2 over V1 is T2 P1 V1 over P2 T1, that's what volume 2 is. And divided by V1 which is the same as multiplying by 1 over v1. v1's cancel and that means the fractional change in volume is temperature up in the atmosphere times the pressure at sea level divided by the pressure way up in the atmosphere times the temperature at sea level. So, temperatures have to be converted into kelvin. 5 degrees Celsius plus 273.15 gives the temperature in kelvin in the upper atmosphere times 1 atmosphere pressure at sea level divided by 0.68 atmospheres of pressure way up there times 20 degrees Celsius plus 273.15. And that gives about 1.4. So, V2 is 1.4 times V1. So, the volume of the balloon up in the atmosphere in the sky is 1.4 times what it was at sea level.
