What is the rms speed of nitrogen molecules contained in an - volume at 2.9 atm if the total amount of nitrogen is 2100 mol?
In order to watch this solution you need to have a subscription.
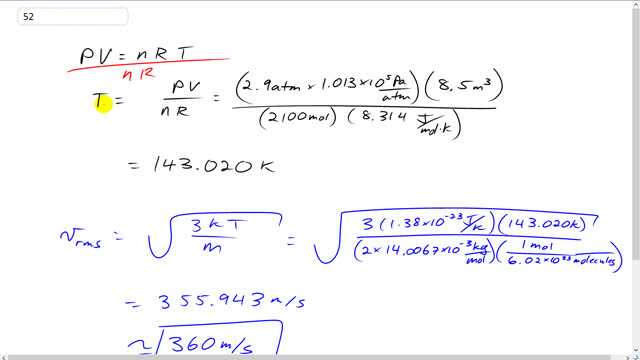
This is Giancoli Answers with Mr. Dychko. To figure out the rms speed, the first thing we need to know is what the temperature of this gas is. So, we use the ideal gas law and rearrange it for T by dividing both sides by n R. So, we have temperature in kelvin as pressure times volume divided by the number of moles times the universal gas constant, R. So, that's 2.9 atmospheres converted into pascals by multiplying by 1.013 times 10 to the 5 pascals per atmosphere. And then multiplied by 8.5 cubic meters divided by 2,100 moles times 8.314 joules per mole kelvin. And that gives is 143.020 kelvin. And the rms speed is going to be square root of 3 times Boltzmann's constant times that absolute temperature divided by the molecular mass. So, that's 3 times 1.38 times 10 to the minus 23 joules per kelvin times 143.020 kelvin that we figured out just before for the temperature divided by 2 times the atomic mass which is 14.0067 times 10 to the minus 3 kilograms per mole. And, sorry, I guess that's the molar mass, not the atomic mass. You can think of it either way, I guess, times by 1 mole for every 6.02 times 10 to 23 molecules, that's Avogadro's number. And this gives us the number of kilograms per molecule which is molecular mass. And that is 360 meters per second for the rms speed.
