A gas is at . To what temperature must it be raised to triple the rms speed of its molecules?
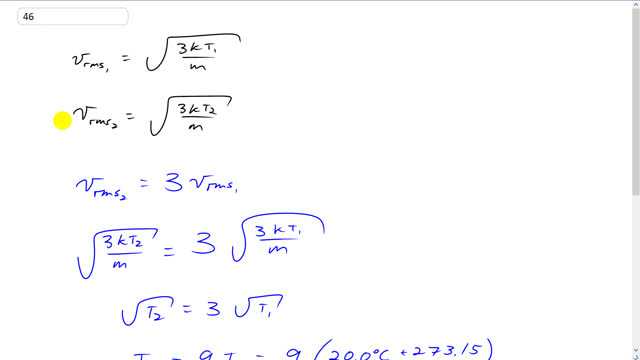
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The rms speed at the first temperature is going to be square root of 3 times Boltzmann's constant times the first temperature, T1, divided by the mass of a single molecule. And then the rms speed of the second temperature is going to be square root of 3 K T2 over m. And the m doesn't need a subscript because it's the same molecule, it's just a different temperature. So, we put a subscript on the T only and we know the V rms2, there's gonna be 3 times the rms speed of the first temperature. And so we substitute for V rms2 that's 3 K T2 over m square rooted. And that equals 3 times V rms1, 3 K T1 over m. And the square, well... Whole bunch of things cancel on both sides, you can multiply both sides by square root m over 3 K, and then that cancels away everything except for this square root 2 or T2 equals 3 times square root T1. And then square both sides. And you get T2 is 9 times T1. So, 9 times the first temperature written in kelvin, that's 20 degree Celsius plus 273.15. And that gives 2638.135 kelvin. And then we subtract away 273.15. And that gives 236.5 degrees Celsius.
