If the humidity in a sealed room of volume at is 65%, what mass of water can still evaporate from an open pan?
In order to watch this solution you need to have a subscription.
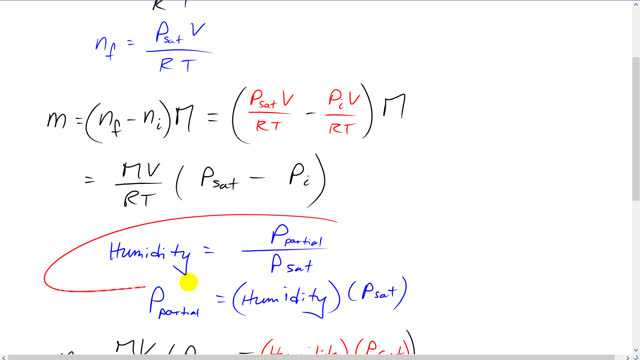
This is Giancoli Answers with Mr. Dychko. The water vapor has some initial pressure in the air, it's the partial pressure, and we're gonna calculate what that partial pressure is in a minute knowing the humidity and knowing the saturated vapor pressure at the known temperature. And this partial pressure represents a certain number of moles of water that are already in the air, and we can solve for it by dividing both sides by R T. Now, the maximum number of moles and final after some more evaporates is going to equal the saturated vapor pressure times V over R T. And the difference in the number of moles multiplied by the molar mass gives the mass of water that can still go into the air before it starts to condense out. So, we substitute in for the number of moles final and the number of moles initial. We have P saturated times v over R T and the P initial, the partial pressure times V over R T, all times the molar mass. And we can factor out the V R and T, multiplied by M. And you get all that times P saturated minus P initial. So, humidity is partial pressure divided by saturated vapor pressure. And we can solve for partial pressure by multiplying both sides by P saturated. And this partial pressure is the initial pressure of the water. And so we substitute humidity times P saturated which then becomes, we have p saturated as a common factor now and that gets factored out. So, we have M V P saturated over R T times 1 minus the humidity. So, the molar mass of water is 2 times the molar mass of hydrogen read from the periodic table of elements in the back cover of the textbook, and we multiply it by 10 to the minus 3 kilograms per mole plus the molar mass of oxygen, written as times 10 to minus 3 kilogram per mole times the volume of the room 420 cubic meters times the saturated vapor pressure at 20 degrees which is 2.33 times 10 to the 3 pascals. And we read this number off of the table 13-3. And then go 1 minus 0.65, this being 65% is the humidity, divided by ideal gas constant, 8.314 joules per mole kelvin, multiplied by the temperature in kelvin, 25 degrees Celsius plus 273.15. And this gives about 2.5 kilograms more water can still be put into the air at this temperature.
