If the pressure in a gas is tripled while its volume is held constant, by what factor does change?
In order to watch this solution you need to have a subscription.
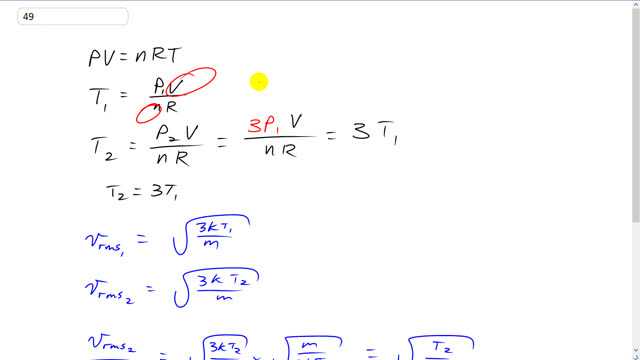
This is Giancoli Answers with Mr. Dychko. We can use the ideal gas law with temperature and pressure are changeable. The number of moles and the volume are not gonna be changing because we're told that its volume is held constant and it doesn't mention that the container is sealed but that's just assumed. So, the number of moles will not change either. So, we can solve this for T by dividing both sides by n R. And T in the first case going to be P1 V over n R. And T in the second case will be T2 is going to be P2 over or times V over n R and P2 we're told is 3 times the first pressure. So, the second pressure is 3 times the first pressure. So, we have 3 times P1 V over n R but P1 V over n R is T1. So, we'll substitute that in so, T2 is 3 times T1. And V rms in the first case is going to be square root of 3 K temperature 1 divided by m and V rms in the second case is the same thing with the T2 instead of T1. And we divide them to find the factor by which they are different. And so that means 3 K T2 over m square rooted multiplied by the reciprocal of V rms1. So, multiplied by m over 3 K T1 square rooted which gives a bunch of things cancelling. And square root T2 over T1. So, the factor by which the rms speed and the case 2 is different from the rms speed in case 1, is square root of 3 T1 because that's what we figured out from this ideal gas law that T2 is 3 times T1 when you increase the pressure by 3 times while holding volume constant. And the T1's cancel. And we're left with square root of 3.
