Is a gas mostly empty space? Check by assuming that the spatial extent of the gas molecules in air is about so one gas molecule occupies an approximate volume equal to . Assume STP.
In order to watch this solution you need to have a subscription.
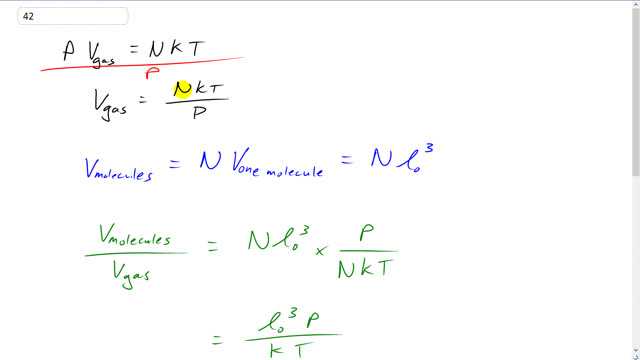
This is Giancoli Answers with Mr. Dychko. Pressure times the volume of the gas equals the number of molecules times Boltzmann's constant times the temperature. And we can divide both sides by the pressure to get the volume of the gas N K T over P. Now, the volume occupied by the molecules themselves is going to be the number of molecules times the volume of 1 molecule volume per molecule. So, and we're told the volume per molecule is l naught cubed. And so to find the fraction of the total volume that the molecules take. We'll take volume of the molecules divided by the volume of the gas. And so that's an l naught cubed, volume of the molecules, divided by V gas but we'll multiply it by its reciprocal. So, multiply it by P over N K T. And the N's cancel which is good because we don't know how many molecules were dealing with. And we have l naught cubed times pressure over K T. And we have to assume standard temperature and pressure. So, that means we have 0.3 nanometers and nano is times 10 to the minus 9. So, we have 0.3 times 10 to the minus 9 meters cubed times 1.013 times 10 to the 5 pascals atmospheric pressure divided by 1.38 times 10 to the negative 23 joules per kelvin, Boltzmann's constant times 273 kelvin, standard temperature. And that gives 7.3 times 10 to the minus 4, that's the fraction of the volume of the gas volume that's occupied by the molecules themselves. So, gases are mostly empty space. So, the molecules account for about 0.07% after you multiply this by 100 to turn it into a percent, 0.07% of the volume of the gas is occupied by the molecules themselves.
