Problem 2
Q
How many atoms are there in a 3.4-g copper coin?
A
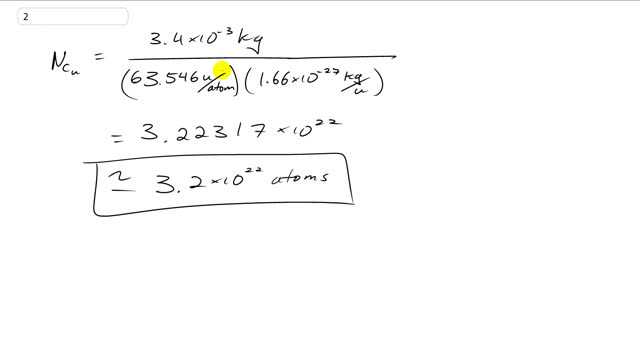
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
This is Giancoli Answers with Mr. Dychko. The number of copper atoms is the mass of copper, 3.4 times 10 to the minus 3 kilograms after changing grams and kilograms by multiplying by 10 to the minus 3 and divided by the atomic mass of copper, which you can find in the periodic table of elements in the back cover of your textbook, 63.546 atomic mass units per atom. And then convert that into kilograms, we're multiplying by 1.66 times 10 to the minus 27 kilograms per atomic mass unit. And we'll end up with 3.2 times 10 to the 22 atoms.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
