
A storage tank contains 21.6 kg of nitrogen at an absolute pressure of 3.45 atm. What will the pressure be if the nitrogen is replaced by an equal mass of at the same temperature?
In order to watch this solution you need to have a subscription.
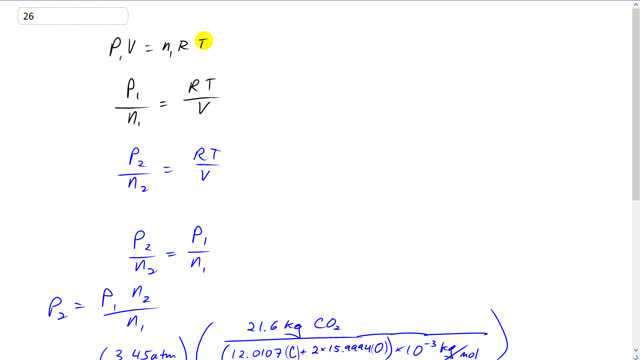
This is Giancoli Answers with Mr. Dychko. The first pressure when the container contains a nitrogen times the continuous volume equals the number of moles of nitrogen times the universal gas constant times the temperature written in kelvin. And we can divide both sides by v. And then also divide both sides by n1. So, v n1 here and v n1 there. And on the right hand side we're left with R T over v and on the left side we're left with p1 over n1. And reason not useful is because we can rewrite this with the quantities when it is filled with carbon dioxide, in which case we have some different pressure, p2, and some different number of moles because it's the same mass of carbon dioxide but the molar mass of carbon dioxide is different, so, it'd be a different number of molecules or a different number of moles. And it's still going to equal the same or R T over v however because the temperature we're told is the same and the container of volume will be the same as well. And so since these both equal R T over v then these ratios will equal each other. So, p2 over n2 equals p1 over n1. And we can solve for the pressure in the second case by going p1 over n1 times n2. So, p1 is 3.45 atmospheres, that's the pressure when there's nitrogen in there. And we'll multiply it by the number of moles of carbon dioxide. So, that's 21.6 kilograms of carbon dioxide divided by the molar mass expressed in kilograms per mole. So, to find this you look in the periodic table in the back cover of the textbook, and it says 12.0107 below carbon and that is the number of grams per mole. And we multiply it by 10 to the minus 3 to get kilograms per mole. And carbon dioxide also has 2 oxygen atoms in it. So, we go 2 times 15.9994 grams per mole for oxygen. And so then this total is the molar mass in grams per mole. And then we multiply by 10 to the minus 3 get kilograms per mole and the kilograms cancel on top and bottom. And it's the same process for nitrogen. Same mass for nitrogen, 21.6 kilograms, divided by 2 times 14.0067 grams per mole. And then multiplied by 10 to the minus 3 kilograms per mole and we end up with 2.20 atmospheres. Since we left this pressure in units of atmospheres, our answer is also going to have units of atmospheres.