You buy an “airtight” bag of potato chips packaged at sea level, and take the chips on an airplane flight. When you take the potato chips out of your “carry-on”bag, you notice it has noticeably “puffed up.” Airplane cabins are typically pressurized at 0.75 atm, and assuming the temperature inside an airplane is about the same as inside a potato chip processing plant, by what percentage has the bag “puffed up” in comparison to when it was packaged?
In order to watch this solution you need to have a subscription.
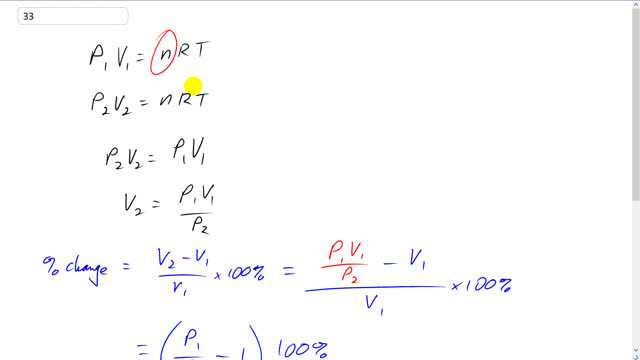
This is Giancoli Answers with Mr. Dychko. In the chip packaging plant there was some pressure there, P1, and some initial volume of the bag V1 equals the number of moles of the air inside the bag times the universal gas constant, R, times the temperature. And we're told of the temperature and the chip packaging plant is about the same as the temperature on the plane and so it does not need a subscript, it's just T. On the plane however the pressure is different and so the volume will also be different. So, we have P2 V2. Now, these pressure volume products both equal n R T and so they're equal to each other. So, P2 V2 equals P1 V1. And we can divide both sides by P2 to solve for the volume on the plane, V2, that's going to be P1 V1 over P2. So, the percent change is going to be the difference in volumes divided by the original volume and that's going to be V2 minus V1 over V1 times 100%, and V2 we just figured out is P1 V1 over P2. And it's still minus V1 all over V1. V1 is a common factor on top that you can factor out and then cancel with a V1 on the bottom. And so we end up with P1 over P2 minus 1 is going to be, times 100%, is going to be the percent change. This minus 1 comes from the fact that, well, this turns into V1 times bracket P1 over P2 minus 1. And then the V1's cancel. OK. So, we have 1 atmosphere divided by 0.75 atmospheres, cabin pressure on the plane, minus 1 times 100%, gives about 33% I s gonna be the change in volume, that's positive. And so it's gonna be 33% larger on the plane than it was in the chip packaging plant.
