Problem 60
Q
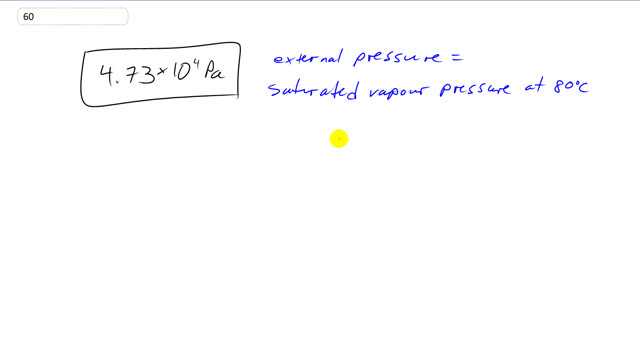
What is the air pressure at a place where water boils at ?
A
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
This is Giancoli Answers with Mr. Dychko. When the external air pressure equals the saturated vapor pressure at 80 degrees then you have boiling occurring. And when you look in this table 13-3 on page 379, you see the saturated vapor pressure for water at 80 degrees Celsius is 4.73 times 10 to the 4 pascals. And so since boiling is occurring at this time, that means the external pressure is the same.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
