
A sealed metal container contains a gas at and 1.00 atm. To what temperature must the gas be heated for the pressure to double to 2.00 atm? (Ignore expansion of the container.)
In order to watch this solution you need to have a subscription.
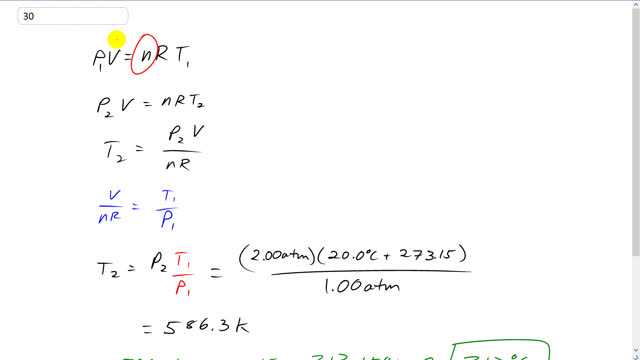
This is Giancoli Answers with Mr. Dychko. Because we have a sealed metal container we know that the n is going to be constant because it's sealed. And since the container is metal, it's rigid. And so v is constant as well. And we don't need any subscripts on these for the two scenarios. So, at the first pressure we have p1 times v equals n R T1. And then when the pressure is increased to 2 atmospheres, we have that second pressure times the same volume equals the same quantity, same number of moles of gas times r times a different temperature, t2. And we can rearrange this to solve for t2 and by dividing both sides by n R. And we get t2 is p2 over v, p2 times v over n R. And then from here, we can see that v over n R is t1 over p. If you take this and divide both sides by p1 n R, you end up with t1 over p1. And then it also equals v over n R on the other side. So, v over n R is t1 over p1 based on this first equation. And that gets substituted into the second equation, replacing this with t1 over p1. So, t2 is p2, from here, times t1 over p1 because that's what v over n R is. And that's 2 atmospheres times the temperature in kelvin, 28 degrees Celsius plus 273.15. And divided by the original pressure of 1 atmosphere. And that gives 286.3 kelvin. And minus 273.15 to get the answer in degrees Celsius of 313.