What speed would a 1.0-g paper clip have if it had the same kinetic energy as a molecule at ?
Note: the video incorrectly shows adding to 273 in order to convert to Kelvin. It should add instead. It turns out that the final answer is the same to two significant figures since the percent difference between 293 vs 295 is so small, so the only thing to note here is that the work shown in the video should say 22 rather than 20.
In order to watch this solution you need to have a subscription.
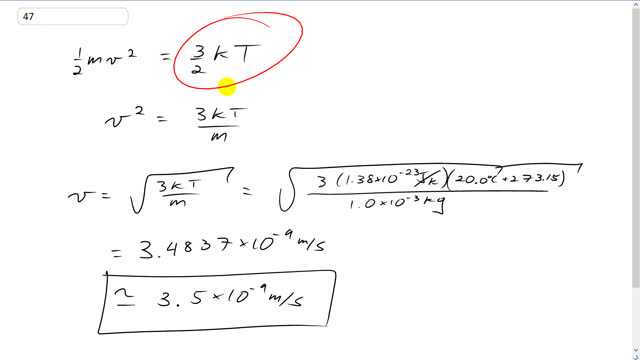
This is Giancoli Answers with Mr. Dychko. We'll set the kinetic energy of the 1 gram paperclip 1/2 mass times its velocity squared equal to the average kinetic energy of a single molecule at 22 degrees Celsius. And so we'll multiply both sides by 2 and so the 2's cancel on both sides and divide both sides by m. And you get V squared is 3 K T over m. And then square root both sides and you get V is square root of 3 K T over m. So, that's square root of 3 times Boltzmann's constant, 1.38 times 10 to the minus 23 joules per kelvin times the temperature written in kelvin, 20 degrees Celsius plus 273.15 divided by the mass of the paperclip which is 1 gram or 1 times 10 to the minus 3 kilograms. And that gives about 3.5 times 10 to the negative 9 meters per second. So, we expected a small number here because the mass of a single molecule is really small, and whereas the mass of the paperclip, you know, 1 gram doesn't seem very big but it's really big compared to a molecule. So, we expected a small speed for the paperclip in order to make the same kinetic energy as a single molecule.
i think the temperature is 22° not 20°?
Hi elkinsk, thank you for noticing that! You're quite correct that the temperature should be instead of . It turns out that the final answer is the same in either case since the 22 or 20 get added to 273 in order to convert to Kelvin, and the percent difference between 295 and 293 is small. I'll put a note for other students about this, and thanks again.
Best wishes with your studies,
Mr. Dychko
