Problem 55
Q
exists in what phase when the pressure is 35 atm and the temperature is (Fig. 13–23)?
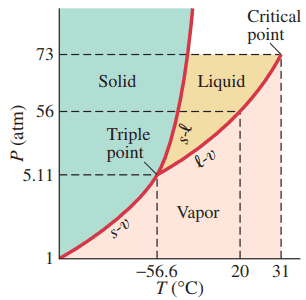
Figure 13-23 Phase diagram for carbon dioxide.
A
only gas is possible
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
This is Giancoli Answers with Mr. Dychko. This is the phase diagram for carbon dioxide and it has a critical temperature of 31 degrees Celsius. So, that means anything beyond that, such as 35 degrees Celsius, is going to be in a gas phase. And that's the only phase that's possible at temperatures beyond the critical temperature.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
