
A tire is filled with air at to a gauge pressure of 230 kPa. If the tire reaches a temperature of , what fraction of the original air must be removed if the original pressure of 230 kPa is to be maintained?
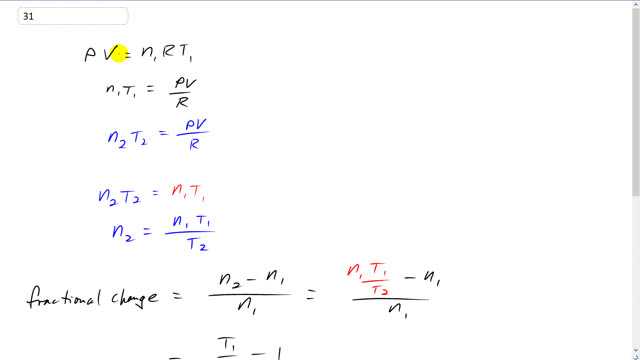
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The pressure in this tire is constant because it's 230 kilopascals in both cases. And we assume the tire is rigid so, it's volume is constant, I mean, that's not precisely true. But let's suppose that it is. And the number of moles of air in the tire however will change because we're told to remove some of the air. And then so that gets a subscript 1 and then times universal gas constant times the original temperature. So, n1 T1 equals P V over R if you divide both sides by R. And then in the second case when the temperature has increased to 38 degrees Celsius, we're gonna have some new number of moles because we have to remove some of them in order to maintain the same pressure which does not have a subscript because it's constant and it's the same in both cases times the same volume divided by R. Now, since both of these products equal the same thing, they're equal to each other. So, n2 T2 equals n1 T1 in which case n2 is n1 T1 over T2. So, the fractional change is going to be n2 minus n1. So, that's the change in the number of moles divided by the original number of moles. And n2 is n1 T1 over T2, and minus n1 all over n1 n1 is a common factor on top and bottom now, so it cancels, and the fractional changes is T1 divided by T2 minus 1. The temperatures have to be expressed in kelvin. So, it's 15 degrees Celsius plus 273.15 divided by 38 degrees Celsius plus 273.15 minus 1, gives negative 0.0739. The negative means the fractional changes resulted in a loss in the number of moles, reduced number of moles here. And we can multiply by 100 to turn into a percent. So, there's 7.4% of the gas is removed. And this is as a percentage of about the number of moles. Sometimes to deal with gases seems confusing whether to talk about should I express the number of milliliters or cubic meters removed or should it be the number of kilograms removed or the number of moles removed. In this case it's the number of moles removed.