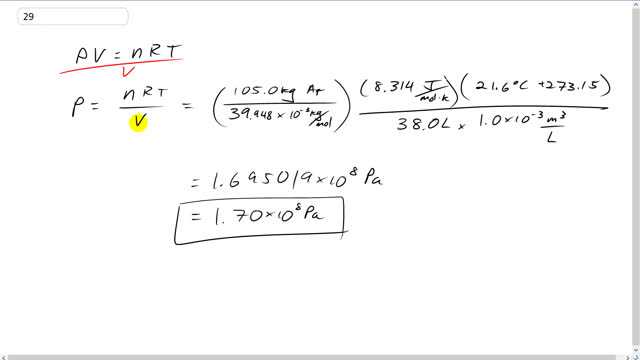
What is the pressure inside a 38.0-L container holding 105.0 kg of argon gas at ?
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. We solved the ideal gas law for pressure by dividing both sides by volume. An your pressure is the number of moles times the universal gas constant times the temperature in kelvin divided by the volume in cubic meters. There's a lot of unit conversion involved here. First of all we have to turn the argon kilograms into a number of moles of argon. So, 105 kilograms divided by 39.948 times 10 to the minus 3 kilograms per mole. And this number comes from the periodic table of elements in the back cover of the textbook. And all those numbers there for atomic mass can be thought of as grams per mole. And we're multiplying by 10 to the minus 3 to turn into kilograms per mole. And this gives the number of moles of argon here. Then times by universal gas constant, 8.314 joules per mole kelvin and then times by 21.6 degrees Celsius plus 273.15 to convert this temperature into absolute temperature or temperature in kelvin. And divide that by 308.0 liters times 10 to the minus 3 cubic meters per liter. And we end up with 1.70 times 10 to the 8 pascals.
